Learning Objectives Define electrolyte. Define nonelectrolyte. List common electrolyets and nonelectrolytes. Why do runners worry about losing electrolytes? Joggers by Tony Alter ( Flickr: Tobyotter ). Millions of people in the world jog for exercise. For the most part, jogging can be a healthy way to stay fit.
MetaLyte™ Horse Electrolyte Powder | Perfect Products EQ
H₂C₂O₄ (aq) + 2NaOH (aq) → Na₂C₂O₄ (aq) + 2H₂O (l) A. combustion. B. oxidation-reduction. C. acid-base neutralization. D. precipitation. B. 965mmHg. An open ended mercury manometer is used to measure the gas pressure in a glass bulb as shown below (the difference in height is 225mm, the tube on the gas side is farther down (by

Source Image: bevmo.com
Download Image
Weak Electrolytes. Weak electrolytes partially ionize in water. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice, around 1% to 10% of a weak electrolyte breaks into ions. Examples: Weak acids and weak bases are weak electrolytes. Most nitrogen-containing molecules are weak electrolytes.

Source Image: purekick.com
Download Image
What Are Electrolytes? – O.R.S Hydration
Potassium, magnesium, and phosphate ions are the predominant electrolytes in a. plasma, b. interstitial fluid, c. intracellular fluid. Sodium balance is regulated primarily by control of amount (s) a. ingested, b. excreted in urine, c. lost in perspiration, d. lost in feces. Answer questions 5 through 10 by choosing responses from the following

Source Image: medicalnewstoday.com
Download Image
Which Of The Following Is Not An Electrolyte
Potassium, magnesium, and phosphate ions are the predominant electrolytes in a. plasma, b. interstitial fluid, c. intracellular fluid. Sodium balance is regulated primarily by control of amount (s) a. ingested, b. excreted in urine, c. lost in perspiration, d. lost in feces. Answer questions 5 through 10 by choosing responses from the following
Home / Health Library / Diagnostics & Testing / Electrolytes Electrolytes Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in water. They help your body regulate chemical reactions, maintain the balance between fluids inside and outside your cells, and more.
Which drinks contain electrolytes and how to make them at home
Jun 30, 2023The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). These are nutritionally called macrominerals. Electrolyte balance is crucial to many body functions.
Nuun Daily- Essential Electrolytes – Nuun Hydration

Source Image: nuunlife.com
Download Image
Endocrine System Anatomy and Physiology – Nurseslabs
Jun 30, 2023The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). These are nutritionally called macrominerals. Electrolyte balance is crucial to many body functions.
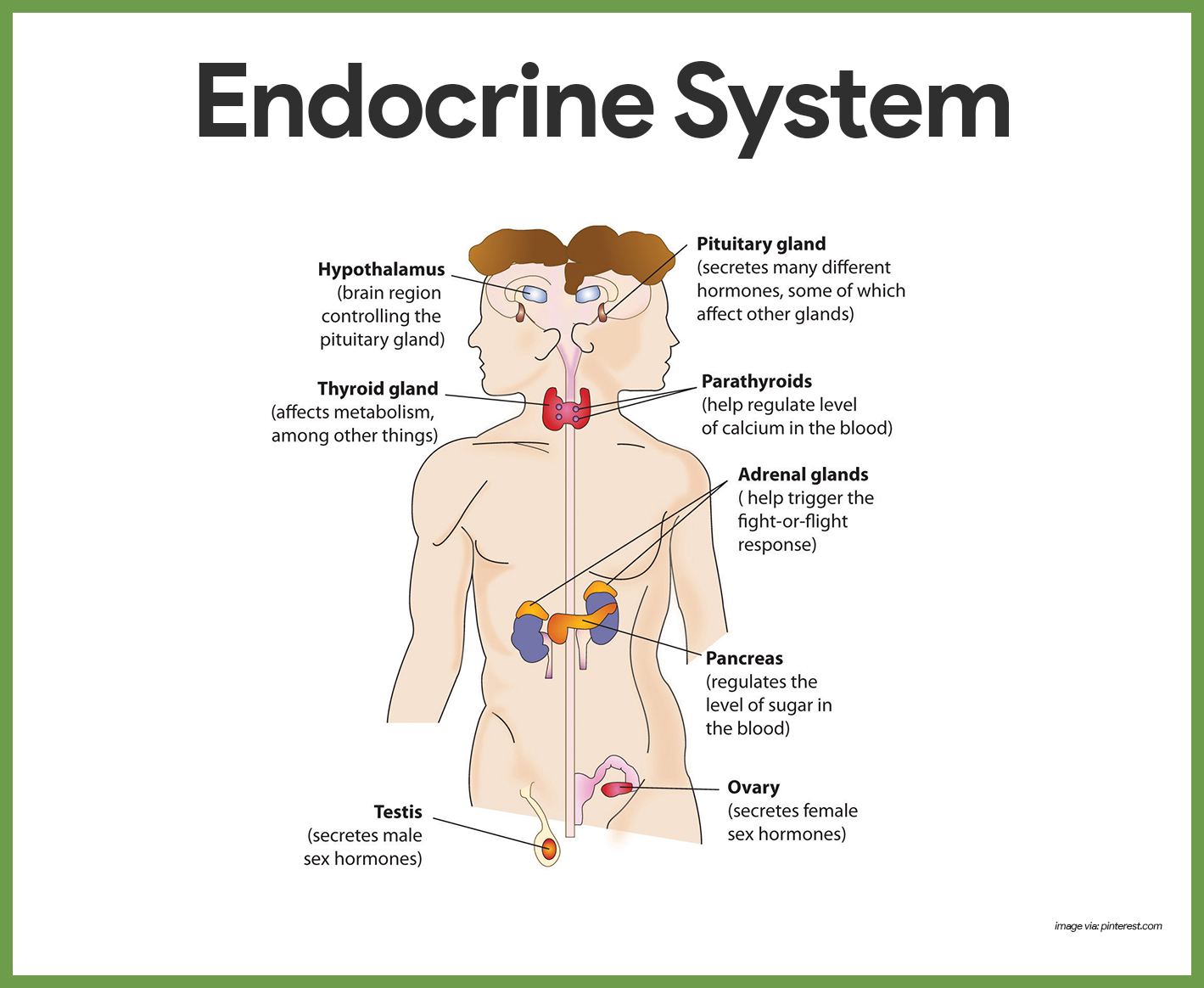
Source Image: nurseslabs.com
Download Image
MetaLyte™ Horse Electrolyte Powder | Perfect Products EQ
Learning Objectives Define electrolyte. Define nonelectrolyte. List common electrolyets and nonelectrolytes. Why do runners worry about losing electrolytes? Joggers by Tony Alter ( Flickr: Tobyotter ). Millions of people in the world jog for exercise. For the most part, jogging can be a healthy way to stay fit.

Source Image: perfectproductseq.com
Download Image
What Are Electrolytes? – O.R.S Hydration
Weak Electrolytes. Weak electrolytes partially ionize in water. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice, around 1% to 10% of a weak electrolyte breaks into ions. Examples: Weak acids and weak bases are weak electrolytes. Most nitrogen-containing molecules are weak electrolytes.

Source Image: orshydration.com
Download Image
Calf Res-Q® Rehydration Supplement from Alltech
Figure 9.4.2.1 9.4.2. 1: Solutions of nonelectrolytes, such as ethanol, do not contain dissolved ions and cannot conduct electricity. Solutions of electrolytes contain ions that permit the passage of electricity. The conductivity of an electrolyte solution is related to the strength of the electrolyte. Water and other polar molecules are

Source Image: store.alltech.com
Download Image
Anti-inflammatory Diet* (Healthy Foods For All) | Facebook
Potassium, magnesium, and phosphate ions are the predominant electrolytes in a. plasma, b. interstitial fluid, c. intracellular fluid. Sodium balance is regulated primarily by control of amount (s) a. ingested, b. excreted in urine, c. lost in perspiration, d. lost in feces. Answer questions 5 through 10 by choosing responses from the following

Source Image: facebook.com
Download Image
Lemon Lime Hydration With Electrolytes Vegan Supplements – 17oz/30ct Stick Packs – Up & Up™ : Target
Home / Health Library / Diagnostics & Testing / Electrolytes Electrolytes Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in water. They help your body regulate chemical reactions, maintain the balance between fluids inside and outside your cells, and more.

Source Image: target.com
Download Image
Endocrine System Anatomy and Physiology – Nurseslabs
Lemon Lime Hydration With Electrolytes Vegan Supplements – 17oz/30ct Stick Packs – Up & Up™ : Target
H₂C₂O₄ (aq) + 2NaOH (aq) → Na₂C₂O₄ (aq) + 2H₂O (l) A. combustion. B. oxidation-reduction. C. acid-base neutralization. D. precipitation. B. 965mmHg. An open ended mercury manometer is used to measure the gas pressure in a glass bulb as shown below (the difference in height is 225mm, the tube on the gas side is farther down (by
What Are Electrolytes? – O.R.S Hydration Anti-inflammatory Diet* (Healthy Foods For All) | Facebook
Figure 9.4.2.1 9.4.2. 1: Solutions of nonelectrolytes, such as ethanol, do not contain dissolved ions and cannot conduct electricity. Solutions of electrolytes contain ions that permit the passage of electricity. The conductivity of an electrolyte solution is related to the strength of the electrolyte. Water and other polar molecules are