2. When a solution undergoes a chemical reaction, the concentration of H+ ions may change, leading to a change in pH. 3. The magnitude of the pH change depends on the strength of the acid or base involved in the reaction, as well as the concentration of the reactants and products. 4. If a solution undergoes a reaction that involves a strong
Percentage of Businesses That Fail [Updated Jan 2024]
when a solution showed the greatest change in PH. Why does a happen? Which of the following solutions would show the greatest change in pH upon the addition of 10.0 mL of 1.0 M NaOH to 1.0 L of the solution?

Source Image: nytimes.com
Download Image
Dec 7, 2023It seems like the context or specific solutions you’re referring to is missing in your question. The change in pH of a solution can be influenced by various factors, such as the addition of acids, bases, or other chemicals. Without specific information

Source Image: medicalnewstoday.com
Download Image
Solved Q4 Which solution(s) showed the greatest change in | Chegg.com
Dec 6, 2022Answer No one rated this answer yet — why not be the first? 😎 AladdinA123 report flag outlined Final answer: The solution that showed the greatest change in pH was the one where the acid concentration increased to 0.10M, causing a significant decrease in pH from 7 to 1.

Source Image: business.pinterest.com
Download Image
Which Solutions Showed The Greatest Change In Ph Why
Dec 6, 2022Answer No one rated this answer yet — why not be the first? 😎 AladdinA123 report flag outlined Final answer: The solution that showed the greatest change in pH was the one where the acid concentration increased to 0.10M, causing a significant decrease in pH from 7 to 1.
Aug 18, 2023Question: If you add acid to a buffer, how will the pH change? Answer: pH slightly decreases. Question: If you add base to a buffer, how will the pH change? Answer: pH slightly increases. Question: Which solution(s) showed the greatest change in pH? Why? Answer: The water and NaCl solution; not buffers. Question: Which solutions(s) showed
Marketing on Pinterest | Pinterest Business
The pH scale is used to rank solutions in terms of acidity or basicity (alkalinity). Since the scale is based on pH values, it is logarithmic, meaning that a change of 1 pH unit corresponds to a ten-fold change in H + ion concentration. The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although
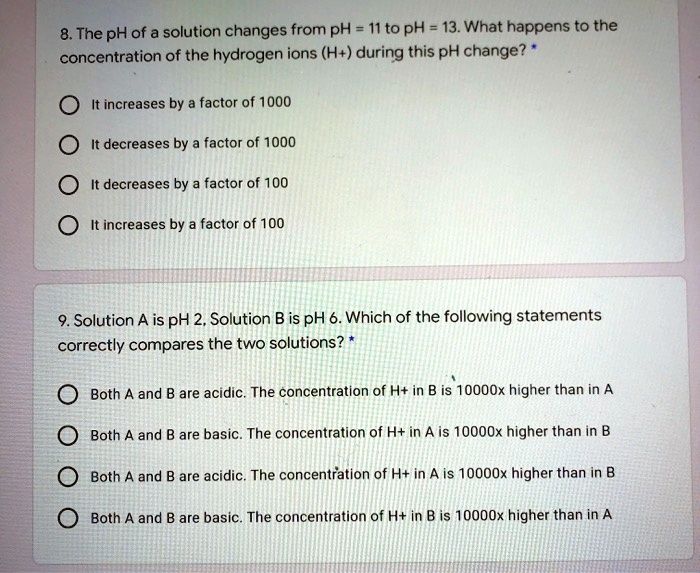
SOLVED: The pH of a solution changes from pH 11 to pH = 13. What happens to the concentration of the hydrogen ions (H+) during this pH change? It increases by a
Source Image: numerade.com
Download Image
Claim your website | Pinterest Business help
The pH scale is used to rank solutions in terms of acidity or basicity (alkalinity). Since the scale is based on pH values, it is logarithmic, meaning that a change of 1 pH unit corresponds to a ten-fold change in H + ion concentration. The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although

Source Image: help.pinterest.com
Download Image
Percentage of Businesses That Fail [Updated Jan 2024]
2. When a solution undergoes a chemical reaction, the concentration of H+ ions may change, leading to a change in pH. 3. The magnitude of the pH change depends on the strength of the acid or base involved in the reaction, as well as the concentration of the reactants and products. 4. If a solution undergoes a reaction that involves a strong
![Percentage of Businesses That Fail [Updated Jan 2024]](https://cdn.shopify.com/s/files/1/0070/7032/articles/Fail_Rates_of_Businesses_That_Started_in_2018.png?format=jpg&quality=90&v=1705405847)
Source Image: shopify.com
Download Image
Solved Q4 Which solution(s) showed the greatest change in | Chegg.com
Dec 7, 2023It seems like the context or specific solutions you’re referring to is missing in your question. The change in pH of a solution can be influenced by various factors, such as the addition of acids, bases, or other chemicals. Without specific information

Source Image: chegg.com
Download Image
Garlic Butter Shrimp – Damn Delicious
Science Chemistry Q4 Which solution (s) showed the greatest change in pH? Why? Q5 Which solutions (s) showed little or no change in pH? Why? Q6 Normally, the pH of the human body is fixed in a very narrow range between 7.35 and 7.45. A patient with an acidotic blood pH of 7.3 may be treated with an alkali such as sodium hydrogen carbonate.

Source Image: damndelicious.net
Download Image
How To Use Pinterest For Business: The Ultimate Beginner’s Guide
Dec 6, 2022Answer No one rated this answer yet — why not be the first? 😎 AladdinA123 report flag outlined Final answer: The solution that showed the greatest change in pH was the one where the acid concentration increased to 0.10M, causing a significant decrease in pH from 7 to 1.

Source Image: later.com
Download Image
China building airstrip on disputed island in South China Sea, satellite images suggest
Aug 18, 2023Question: If you add acid to a buffer, how will the pH change? Answer: pH slightly decreases. Question: If you add base to a buffer, how will the pH change? Answer: pH slightly increases. Question: Which solution(s) showed the greatest change in pH? Why? Answer: The water and NaCl solution; not buffers. Question: Which solutions(s) showed

Source Image: nbcnews.com
Download Image
Claim your website | Pinterest Business help
China building airstrip on disputed island in South China Sea, satellite images suggest
when a solution showed the greatest change in PH. Why does a happen? Which of the following solutions would show the greatest change in pH upon the addition of 10.0 mL of 1.0 M NaOH to 1.0 L of the solution?
Solved Q4 Which solution(s) showed the greatest change in | Chegg.com How To Use Pinterest For Business: The Ultimate Beginner’s Guide
Science Chemistry Q4 Which solution (s) showed the greatest change in pH? Why? Q5 Which solutions (s) showed little or no change in pH? Why? Q6 Normally, the pH of the human body is fixed in a very narrow range between 7.35 and 7.45. A patient with an acidotic blood pH of 7.3 may be treated with an alkali such as sodium hydrogen carbonate.